Below is a one-stop shop for the key points you'll need to know for Monday's test. (It's all just a repeat of the review.)
Extra credit will be given for extra practice, so be on the look out for embedded links (bold and italicized)!
Happy studying!
Extra credit will be given for extra practice, so be on the look out for embedded links (bold and italicized)!
Happy studying!
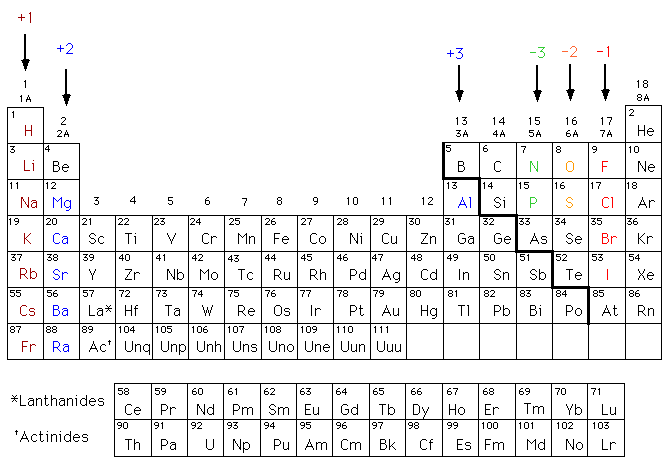
Elements, Valence Electrons, and IONs, oh my!
|
Elements-
Ions- |
Types of Bonds
|
Bonding is an interaction between two (or more) atoms. Being present in the outermost shell, valence electrons are the particles involved in this process. They can be gained, lost, or shared. We focused on the 2 types below.
|
Can you calculate...? |
How many TOTAL electrons will the ION version of an element have?
|
The total number of electrons an atom starts off with equals the number of protons = the atomic number.
Once electrons are gained or lost, this number will change in the same way. For example...
O2+ is oxygen with a 2+ charge (2 electrons were lost)
Oxygen's atomic number is 8, so it had 8 electrons originally.
Total number of electrons = 8 - 2 = 6 electrons are left.
If the ion is negative, you gained electrons, so you'll add the number you gained to your original total.
Once electrons are gained or lost, this number will change in the same way. For example...
O2+ is oxygen with a 2+ charge (2 electrons were lost)
Oxygen's atomic number is 8, so it had 8 electrons originally.
Total number of electrons = 8 - 2 = 6 electrons are left.
If the ion is negative, you gained electrons, so you'll add the number you gained to your original total.
NAMING AND WRITING COMPOUNDS
|
The test will cover 2-element (or binary) compounds.
In order to answer these questions you'll need to use a combo of the knowledge above. How-to steps below: Writing Formulas from a name:
Naming Compounds:
Transition Metals (a special case):
|
More practice? See Kahoot! above.
More help? See the tutorial below.
Your browser does not support viewing this document. Click here to download the document.
|